Molar Heat Capacity Of Water
The molar heat chapters of a chemic substance is the amount of energy that must be added, in the class of rut, to one mole of the substance in order to cause an increase of one unit in its temperature. Alternatively, it is the heat capacity of a sample of the substance divided by the amount of substance of the sample; or also the specific estrus chapters of the substance times its molar mass. The SI unit of tooth oestrus chapters is joule per kelvin per mole, J⋅K−1⋅mol−ane.
Like the specific heat, the measured tooth heat capacity of a substance, specially a gas, may be significantly higher when the sample is allowed to expand as information technology is heated (at constant force per unit area, or isobaric) than when it is heated in a closed vessel that prevents expansion (at constant book, or isochoric). The ratio betwixt the two, however, is the same heat capacity ratio obtained from the corresponding specific oestrus capacities.
This property is most relevant in chemistry, when amounts of substances are often specified in moles rather than past mass or book. The molar heat capacity generally increases with the molar mass, often varies with temperature and pressure, and is dissimilar for each state of matter. For example, at atmospheric pressure, the (isobaric) molar heat chapters of water just to a higher place the melting point is near 76 J⋅K−1⋅mol−1, but that of ice only below that signal is well-nigh 37.84 J⋅Thousand−i⋅mol−one. While the substance is undergoing a phase transition, such as melting or boiling, its molar heat capacity is technically infinite, because the rut goes into changing its country rather than raising its temperature. The concept is not advisable for substances whose precise composition is not known, or whose tooth mass is not well divers, such equally polymers and oligomers of indeterminate molecular size.
A closely related property of a substance is the estrus capacity per mole of atoms, or atom-tooth heat capacity, in which the oestrus capacity of the sample is divided by the number of moles of atoms instead of moles of molecules. Then, for example, the atom-molar estrus capacity of water is 1/3 of its molar oestrus chapters, namely 25.three J⋅K−one⋅mol−1.
In informal chemistry contexts, the molar heat capacity may be chosen just "heat capacity" or "specific heat". However, international standards now recommend that "specific oestrus capacity" always refer to capacity per unit of measurement of mass, to avoid possible confusion.[1] Therefore, the word "molar", not "specific", should ever be used for this quantity.
Definition [edit]
The molar estrus capacity of a substance, which may be denoted by c chiliad, is the heat capacity C of a sample of the substance, divided by the amount (moles) n of the substance in the sample:
- c m
where ΔQ is the amount of heat needed to raise the temperature of the sample past ΔT. Obviously, this parameter cannot be computed when n is not known or defined.
Like the oestrus chapters of an object, the molar heat capacity of a substance may vary, sometimes substantially, depending on the starting temperature T of the sample and the pressure level P applied to information technology. Therefore, it should be considered a function c m(P,T) of those two variables.
These parameters are usually specified when giving the molar heat capacity of a substance. For case, "H2O: 75.338 J⋅K−i⋅mol−1 (25 °C, 101.325 kPa)" [2] When not specified, published values of the molar heat capacity c m generally are valid for some standard conditions for temperature and force per unit area.
However, the dependency of c m(P,T) on starting temperature and pressure tin can often be ignored in practical contexts, e.thousand. when working in narrow ranges of those variables. In those contexts one can usually omit the qualifier (P,T), and gauge the molar heat chapters past a constant c m suitable for those ranges.
Since the tooth heat capacity of a substance is the specific heat c times the tooth mass of the substance M/N its numerical value is mostly smaller than that of the specific heat. Paraffin wax, for example, has a specific heat of nearly 2500 J⋅K−1⋅kg−1 only a molar estrus capacity of about 600 J⋅Chiliad−one⋅mol−i .
The tooth estrus capacity is an "intensive" property of a substance, an intrinsic feature that does not depend on the size or shape of the amount in consideration. (The qualifier "specific" in front of an all-encompassing belongings ofttimes indicates an intensive holding derived from it.[3])
Variations [edit]
The injection of heat energy into a substance, besides raising its temperature, usually causes an increase in its volume and/or its pressure, depending on how the sample is confined. The option made well-nigh the latter affects the measured tooth heat capacity, even for the same starting force per unit area P and starting temperature T. Two particular choices are widely used:
- If the pressure is kept constant (for example, at the ambient atmospheric pressure), and the sample is allowed to expand, the expansion generates work as the force from the pressure displaces the enclosure. That work must come from the heat energy provided. The value thus obtained is said to be the tooth oestrus capacity at abiding pressure (or isobaric), and is ofttimes denoted c P,thousand, c p,m, c P,chiliad, etc.
- On the other paw, if the expansion is prevented — for instance past a sufficiently rigid enclosure, or by increasing the external pressure level to counteract the internal i — no work is generated, and the heat energy that would have gone into it must instead contribute to the internal energy of the object, including raising its temperature by an actress amount. The value obtained this way is said to be the molar rut capacity at constant volume (or isochoric) and denoted c 5,m, c 5,m, c v,m, etc.
The value of c V,m is always less than the value of c P,grand. This departure is particularly notable in gases where values under constant force per unit area are typically thirty% to 66.7% greater than those at constant volume.[4]
All methods for the measurement of specific heat utilise to molar oestrus capacity also.
Units [edit]
The SI unit of molar oestrus capacity rut is joule per kelvin per mole (J/(Chiliad⋅mol), J/(Yard mol), J K−1 mol−i, etc.). Since an increment of temperature of one degree Celsius is the same as an increment of one kelvin, that is the aforementioned as joule per degree Celsius per mole (J/(°C⋅mol)).
In chemistry, heat amounts are still often measured in calories. Confusingly, ii units with that proper name, denoted "cal" or "Cal", have been usually used to measure amounts of heat:
- the "small-scale calorie" (or "gram-calorie", "cal") is 4.184 J, exactly.
- The "grand calorie" (also "kilocalorie", "kilogram-calorie", or "food calorie"; "kcal" or "Cal") is 1000 small calories, that is, 4184 J, exactly.
When oestrus is measured in these units, the unit of specific heat is usually
-
- ane cal/(°C⋅mol) ("small calorie") = 4.184 J⋅M−i⋅mol−1
- 1 kcal/(°C⋅mol) ("large calorie") = 4184 J⋅K−1⋅mol−1.
The molar heat capacity of a substance has the same dimension as the estrus capacity of an object; namely, L2⋅M⋅T−2⋅Θ−1, or M(Fifty/T)2/Θ. (Indeed, it is the heat chapters of the object that consists of an Avogadro number of molecules of the substance.) Therefore, the SI unit J⋅K−one⋅mol−1 is equivalent to kilogram metre squared per second squared per kelvin (kg⋅mii⋅1000−one⋅s−ii).
Concrete footing [edit]
Monatomic gases [edit]
The temperature of a sample of a substance reflects the boilerplate kinetic energy of its constituent particles (atoms or molecules) relative to its centre of mass. Quantum mechanics predicts that, at room temperature and ordinary pressures, an isolated atom in a gas cannot store any pregnant amount of energy except in the form of kinetic energy. Therefore, when a sure number N of atoms of a monatomic gas receives an input ΔQ of oestrus energy, in a container of fixed volume, the kinetic energy of each atom volition increment by ΔQ/N, independently of the atom's mass. This assumption is the foundation of the theory of ideal gases.
In other words, that theory predicts that the molar oestrus capacity at constant volume c V,m of all monatomic gases volition be the same; specifically,
- c V,m = 3 / 2 R
where R is the ideal gas constant, virtually 8.31446 J⋅K−1⋅mol−i (which is the product of the Boltzmann constant thou B and the Avogadro constant). And, indeed, the experimental values of c V,thousand for the noble gases helium, neon, argon, krypton, and xenon (at i atm and 25 °C) are all 12.5 J⋅Thou−1⋅mol−one, which is 3 / 2 R; fifty-fifty though their diminutive weights range from 4 to 131.
The same theory predicts that the molar heat capacity of a monatomic gas at constant pressure will be
- c P,chiliad = c V,m + R = 5 / 2 R
This prediction matches the experimental values, which, for helium through xenon, are xx.78, 20.79, 20.85, xx.95, and 21.01 J⋅Thousand−1⋅mol−1, respectively;[five] [6] very shut to the theoretical five / 2 R = xx.78 J⋅Chiliad−1⋅mol−1.
Therefore, the specific heat (per unit of measurement of mass, not per mole) of a monatomic gas will be inversely proportional to its (adimensional) relative diminutive mass A. That is, approximately,
- c V = (12470 J⋅Thou−i⋅kg−ane)/A c P = (20786 J⋅K−1⋅kg−1)/A
Polyatomic gases [edit]

Vibration of atoms in the molecule and rotation of the molecule store some of the energy (transferred to the molecule every bit heat) that otherwise would contribute to the molecule'south kinetic energy.
Degrees of freedom [edit]
A polyatomic molecule (consisting of ii or more atoms bound together) can shop heat energy in other forms besides its kinetic energy. These forms include rotation of the molecule, and vibration of the atoms relative to its center of mass.
These extra degrees of freedom contribute to the molar heat capacity of the substance. Namely, when heat energy is injected into a gas with polyatomic molecules, only part of it will go into increasing their kinetic energy, and hence the temperature; the rest will go to into those other degrees of freedom. Thus, in order to achieve the same increase in temperature, more estrus energy will have to exist provided to a mol of that substance than to a mol of a monatomic gas. Substances with high diminutive count per molecule, like octane, can therefore have a very big estrus chapters per mole, and yet a relatively small-scale specific heat (per unit mass).[7] [8] [9]
If the molecule could be entirely described using classical mechanics, and so the theorem of equipartition of energy could be used to predict that each degree of liberty would accept an average free energy in the corporeality of 1 / ii kT, where k is the Boltzmann abiding, and T is the temperature. If the number of degrees of freedom of the molecule is f, then each molecule would be holding, on average, a total free energy equal to 1 / two fkT. And then the molar heat chapters (at constant book) would be
- c Five,m = 1 / 2 fR
where R is the ideal gas abiding. Co-ordinate to Mayer'southward relation, the tooth oestrus capacity at abiding force per unit area would be
- c P,grand = c V,m + R = i / ii fR + R = ane / two (f + 2)R
Thus, each additional degree of freedom volition contribute 1 / 2 R to the tooth heat capacity of the gas (both c V,grand and c P,k).
In particular, each molecule of a monatomic gas has only f = 3 degrees of liberty, namely the components of its velocity vector; therefore c Five,m = 3 / 2 R and c P,1000 = 5 / 2 R.[ten]
Rotational modes of a diatomic molecule [edit]
For example, the tooth heat capacity of nitrogen Due north
2 at constant volume is twenty.6 J⋅K−1⋅mol−1 (at 15 °C, i atm), which is 2.49R.[xi] From the theoretical equation c Five,m = 1 / 2 fR, i concludes that each molecule has f = v degrees of freedom. These turn out to be three degrees of the molecule's velocity vector, plus ii degrees from its rotation near an axis through the heart of mass and perpendicular to the line of the two atoms. The degrees of liberty due to translations and rotations are called the rigid degrees of liberty, since they do non involve whatever deformation of the molecule.
Because of those ii extra degrees of freedom, the molar estrus capacity c Five,m of N
ii (20.vi J⋅K−one⋅mol−1) is greater than that of an hypothetical monatomic gas (12.5 J⋅G−i⋅mol−1) by a factor of five / three .
Frozen and active degrees of freedom [edit]
Co-ordinate to classical mechanics, a diatomic molecule like nitrogen should accept more than degrees of internal liberty, corresponding to vibration of the 2 atoms that stretch and shrink the bond betwixt them.
For thermodynamic purposes, each direction in which an atom can independently vibrate relative to the residue of the molecule introduces ii degrees of freedom: one associated with the potential energy from distorting the bonds, and one for the kinetic energy of the atom's motion. In a diatomic molecule like Northward
2 , there is only one management for the vibration, and the motions of the two atoms must be opposite merely equal; and so in that location are only two degrees of vibrational freedom. That would bring f up to 7, and c V,grand to three.5R.
The reason why these vibrations are not absorbing their expected fraction of heat energy input is provided by quantum mechanics. According to that theory, the energy stored in each caste of liberty must increment or decrease only in certain amounts (quanta). Therefore, if the temperature T of the system is non high plenty, the boilerplate free energy that would be available for some of the theoretical degrees of liberty (kT/f) may exist less than the corresponding minimum breakthrough. If the temperature is low plenty, that may be the instance for practically all molecules. Ane then says that those degrees of freedom are "frozen". The molar rut chapters of the gas will then be determined only by the "active" degrees of freedom — that, for nigh molecules, can receive enough energy to overcome that quantum threshold.[12]

Abiding-volume specific heat capacity of a diatomic gas (idealised). As temperature increases, rut capacity goes from
three / 2 R (translation contribution only), to
five / two R (translation plus rotation), finally to a maximum of
vii / ii R (translation + rotation + vibration)
For each degree of freedom, at that place is an approximate disquisitional temperature at which information technology "thaws" ("unfreezes") and becomes active, thus beingness able to concord heat energy. For the 3 translational degrees of liberty of molecules in a gas, this critical temperature is extremely small, so they can exist assumed to be always active. For the rotational degrees of freedom, the thawing temperature is normally a few tens of kelvins (although with a very light molecule such as hydrogen the rotational free energy levels will be spaced and then widely that rotational heat capacity may not completely "unfreeze" until considerably higher temperatures are reached). Vibration modes of diatomic molecules generally commencement to activate just well to a higher place room temperature.
In the instance of nitrogen, the rotational degrees of freedom are fully active already at −173 °C (100 Thou, just 23 K above the boiling signal). On the other hand, the vibration modes merely start to get active around 350 K (77 °C) Appropriately, the molar estrus capacity c P,m is nigh constant at 29.ane J⋅Thousand−1⋅mol−1 from 100 M to almost 300 °C. At about that temperature, information technology starts to increase quickly, then it slows downwards again. It is 35.five J⋅K−i⋅mol−1 at 1500 °C, 36.9 at 2500 °C, and 37.5 at 3500 °C.[thirteen] [fourteen] The terminal value corresponds most exactly to the predicted value for f = vii.

Constant-book specific heat chapters of diatomic gases (real gases) between about 200 K and 2000 K. This temperature range is not large plenty to include both breakthrough transitions in all gases. Instead, at 200 Chiliad, all only hydrogen are fully rotationally excited, then all have at least
5 / 2 R heat capacity. (Hydrogen is already below
5 / 2 , but it will require cryogenic conditions for even H2 to autumn to
three / two R). Further, but the heavier gases fully reach
seven / ii R at the highest temperature, due to the relatively modest vibrational free energy spacing of these molecules. HCl and Hii begin to make the transition to a higher place 500 K, merely accept not accomplished it by 1000 Thou, since their vibrational free energy level spacing is too wide to fully participate in estrus capacity, even at this temperature.
The post-obit is a table of some abiding-pressure molar heat capacities c P,m of various diatomic gases at standard temperature (25 °C = 298 K), at 500 °C, and at 5000 °C, and the apparent number of degrees of freedom f * estimated by the formula f * = 2c P,g/R − 2:
| 25 °C | 500 °C | 5000 °C | |||||
|---|---|---|---|---|---|---|---|
| Gas | c P,thousand J⋅M−one⋅mol−1 | f * | c P,m J⋅K−1⋅mol−1 | f * | c P,m J⋅One thousand−one⋅mol−1 | f * | |
| H2 | 28.nine | v.0 | 29.6 | 5.one | 41.two | 7.9 | Not saturated.[15] |
| CO | 29.i | 5.0 | 31.7 | 5.6 | 38.i | seven.ii | Saturated.[xvi] |
| North2 | 29.1 | 5.0 | 31.3 | 5.five | 38.0 | 7.i | Saturated.[xiii] |
| Cl2 | 34.0 | six.2 | 37.0 | half dozen.nine | 39.6 | 7.5 | Max 41.3 at ~3700 C.[17] |
| Brtwo (vapour) | (*)36.4 | half dozen.8 | 37.5 | 7.0 | 39.ii | 7.4 | Max 41.6 at ~3000 C.[eighteen] |
(*) At 59 C (boiling point)
The quantum harmonic oscillator approximation implies that the spacing of energy levels of vibrational modes are inversely proportional to the square root of the reduced mass of the atoms composing the diatomic molecule. This fact explains why the vibrational modes of heavier molecules like Br
two are active at lower temperatures. The tooth heat capacity of Br
2 at room temperature is consistent with f = vii degrees of liberty, the maximum for a diatomic molecule. At high plenty temperatures, all diatomic gases arroyo this value.
Rotational modes of single atoms [edit]
Breakthrough mechanics also explains why the specific heat of monatomic gases is well predicted by the ideal gas theory with the assumption that each molecule is a point mass that has merely the f = 3 translational degrees of freedom.
According to classical mechanics, since atoms have non-zero size, they should besides have 3 rotational degrees of liberty, or f = 6 in total. Likewise, the diatomic nitrogen molecule should have an boosted rotation mode, namely almost the line of the two atoms; and thus accept f = six too. In the classical view, each of these modes should store an equal share of the heat energy.
However, according to quantum mechanics, the energy deviation betwixt the allowed (quantized) rotation states is inversely proportional to the moment of inertia almost the respective axis of rotation. Because the moment of inertia of a single cantlet is exceedingly small, the activation temperature for its rotational modes is extremely high. The same applies to the moment of inertia of a diatomic molecule (or a linear polyatomic one) about the internuclear centrality, which is why that fashion of rotation is non active in full general.
On the other manus, electrons and nuclei can exist in excited states and, in a few exceptional cases, they may be active fifty-fifty at room temperature, or even at cryogenic temperatures.
Polyatomic gases [edit]
The set of all possible ways to infinitesimally readapt the n atoms of a polyatomic gas molecule is a linear infinite of dimension 3n, because each atom tin can be independently displaced in each of 3 orthogonal axis directions. However, some three of these dimensions are just translation of the molecule past an minute displacement vector, and others are just rigid rotations of it by an infinitesimal angle virtually some axis. Still others may correspond to relative rotation of two parts of the molecule virtually a single bail that connects them.
The independent deformation modes—linearly contained ways to actually deform the molecule, that strain its bonds—are but the remaining dimensions of this space. As in the instance diatomic molecules, each of these deformation modes counts as two vibrational degrees of freedom for energy storage purposes: 1 for the potential energy stored in the strained bonds, and one for the extra kinetic energy of the atoms every bit they vibrate nearly the rest configuration of the molecule.
In particular, if the molecule is linear (with all atoms on a straight line), it has simply two non-trivial rotation modes, since rotation about its own axis does not readapt any atom. Therefore, it has iiin − 5 actual deformation modes. The number of free energy-storing degrees of freedom is then f = 3 + 2 + two(3n − 5) = 6n − 5.
For case, the linear nitrous oxide molecule N≡N=O (with n = 3) has 3due north − 5 = 4 independent infinitesimal deformation modes. Two of them can be described as stretching 1 of the bonds while the other retains its normal length. The other two can exist identified which the molecule bends at the fundamental atom, in the 2 directions that are orthogonal to its centrality. In each style, one should assume that the atoms get displaced so that the center of mass remains stationary and there is no rotation. The molecule and then has f = half-dozenn − v = 13 total energy-storing degrees of liberty (3 translational, ii rotational, 8 vibrational). At high plenty temperature, its molar heat capacity then should exist c P,m = 7.5 R = 62.63 J⋅K−one⋅mol−one. For cyanogen N≡C−C≡Northward and acetylene H−C≡C−H (n = iv) the same analysis yields f = nineteen and predicts c P,m = 10.v R = 87.3 J⋅Grand−one⋅mol−1.
A molecule with n atoms that is rigid and non linear has 3 translation modes and 3 non-trivial rotation modes, hence but threenorth − 6 deformation modes. It therefore has f = 3 + 3 + 2(3n − 6) = 6n − half dozen energy-absorbing degrees of freedom (one less than a linear molecule with the aforementioned atom count). Water HiiO (north = 3) is bent in its non-strained land, therefore information technology is predicted to accept f = 12 degrees of freedom.[xix] Methane CHfour (due north = 5) is tridimensional, and the formula predicts f = 24.
Ethane H3C−CH3 (n = viii) has four degrees of rotational freedom: two well-nigh axes that are perpendicular to the key bond, and two more because each methyl group can rotate independently about that bond, with negligible resistance. Therefore, the number of contained deformation modes is threenorth − vii, which gives f = 3 + 4 + 2(3due north − vii) = 6n − 7 = 41.
The following table shows the experimental molar estrus capacities at constant pressure c P,one thousand of the to a higher place polyatomic gases at standard temperature (25 °C = 298 K), at 500 °C, and at 5000 °C, and the credible number of degrees of freedom f * estimated past the formula f * = 2c P,chiliad/R − 2:
| 25 °C | 500 °C | 5000 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Gas | c P,1000 J⋅Yard−1⋅mol−i | f * | c P,chiliad J⋅1000−1⋅mol−ane | f * | c P,chiliad J⋅K−1⋅mol−1 | f * | f | Notes |
| Northward≡North=O | 38.vi | 7.iii | 51.8 | x.5 | 62.0 | 12.9 | 13 | [twenty] |
| N≡C–C≡North | 56.vii | eleven.half dozen | 72.three | 15.4 | 86.7 | xviii.9 | xix | [21] |
| H–C≡C–N | 44.0 | eight.6 | 63.2 | thirteen.two | 92.9 | 20.iii | 19 | [22] |
| H2O | — | — | 38.4 | 7.2 | 59.vii | 12.4 | 12 | [23] |
| CH4 | 35.7 | 6.six | 61.6 | 12.8 | 105.7 | 23.4 | 24 | [24] |
| H3C–CHiii | 52.five | 10.6 | 105.6 | 23.iv | 168.7 | (*)38.6 | 41 | [25] |
(*) At 3000C
Specific estrus of solids [edit]
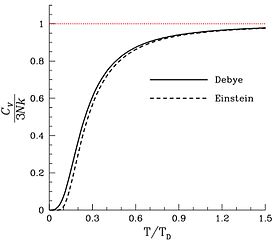
The dimensionless heat chapters divided past three, as a function of temperature equally predicted by the Debye model and by Einstein's earlier model. The horizontal centrality is the temperature divided by the Debye temperature. Annotation that, as expected, the dimensionless heat capacity is cipher at accented zip, and rises to a value of three every bit the temperature becomes much larger than the Debye temperature. The red line corresponds to the classical limit of the Dulong–Petit constabulary
In most solids (but non all), the molecules have a fixed mean position and orientation, and therefore the only degrees of freedom available are the vibrations of the atoms.[26] Thus the specific heat is proportional to the number of atoms (not molecules) per unit of mass, which is the Dulong–Petit law. Other contributions may come from magnetic degrees of freedom in solids, merely these rarely make substantial contributions.[27] and electronic[28] Since each atom of the solid contributes one contained vibration mode, the number of degrees of freedom in n atoms is 6n. Therefore, the heat chapters of a sample of a solid substance is expected to be 3RN a, or (24.94 J/K)N a, where Northward a is the number of moles of atoms in the sample, not molecules. Said another way, the cantlet-molar heat chapters of a solid substance is expected to be iiiR = 24.94 J⋅K−1⋅mol−ane, where "amol" denotes an amount of the solid that contains the Avogadro number of atoms.[29]
It follows that, in molecular solids, the rut chapters per mole of molecules volition unremarkably exist close to 3nR, where n is the number of atoms per molecule.
Thus n atoms of a solid should in principle store twice equally much energy equally n atoms of a monatomic gas. 1 way to look at this result is to find that the monatomic gas can simply store energy as kinetic energy of the atoms, whereas the solid can shop it also equally potential energy of the bonds strained past the vibrations. The atom-tooth oestrus chapters of a polyatomic gas approaches that of a solid as the number due north of atoms per molecule increases.
As in the case f gases, some of the vibration modes will be "frozen out" at low temperatures, especially in solids with light and tightly bound atoms, causing the atom-molar heat capacity to be less than this theoretical limit. Indeed, the atom-molar (or specific) oestrus chapters of a solid substance tends toward zero, as the temperature approaches absolute zilch.
Dulong–Petit law [edit]
As predicted by the above analysis, the heat capacity per mole of atoms, rather than per mole of molecules, is found to be remarkably constant for all solid substances at high temperatures. This relationship was noticed empirically in 1819, and is called the Dulong–Petit law, later its two discoverers.[30] [31] This discovery was an important argument in back up of the atomic theory of affair.
Indeed, for solid metal chemical elements at room temperature, cantlet-molar heat capacities range from about 2.8 R to 3.four R. Large exceptions at the lower terminate involve solids composed of relatively low-mass, tightly bonded atoms, such as beryllium (ii.0 R, only of 66% of the theoretical value), and diamond (0.735 R, only 24%). Those conditions imply larger quantum vibrational energy spacing, thus many vibrational modes are "frozen out" at room temperature. Water ice close to the melting point, also, has an anomalously low heat capacity per atom (1.v R, only 50% of the theoretical value).
At the higher finish of possible heat capacities, oestrus chapters may exceed R by small amounts, due to contributions from anharmonic vibrations in solids, and sometimes a modest contribution from conduction electrons in metals. These are non degrees of freedom treated in the Einstein or Debye theories.
Specific estrus of solid elements [edit]
Sincethe majority density of a solid chemical element is strongly related to its tooth mass, at that place exists a noticeable inverse correlation between a solid's density and its specific estrus chapters on a per-mass basis. This is due to a very approximate trend of atoms of most elements to be about the aforementioned size, despite much wider variations in density and atomic weight. These two factors (constancy of atomic book and constancy of mole-specific oestrus capacity) result in a adept correlation between the volume of any given solid element and its total oestrus capacity.
Another style of stating this, is that the volume-specific estrus capacity (volumetric heat capacity) of solid elements is roughly a constant. The molar volume of solid elements is very roughly abiding, and (even more than reliably) so also is the molar estrus capacity for nigh solid substances. These 2 factors determine the volumetric heat capacity, which every bit a bulk property may exist hitting in consistency. For example, the element uranium is a metal that has a density almost 36 times that of the metallic lithium, merely uranium's specific rut capacity on a volumetric basis (i.due east. per given volume of metallic) is only 18% larger than lithium'southward.
However, the average atomic volume in solid elements is non quite constant, so there are deviations from this principle. For instance, arsenic, which is only fourteen.v% less dense than antimony, has nigh 59% more specific heat capacity on a mass basis. In other words; even though an ingot of arsenic is only well-nigh 17% larger than an antimony 1 of the aforementioned mass, it absorbs well-nigh 59% more heat for a given temperature rise. The heat chapters ratios of the two substances closely follows the ratios of their molar volumes (the ratios of numbers of atoms in the same volume of each substance); the departure from the correlation to elementary volumes, in this example, is due to lighter arsenic atoms being significantly more closely packed than antimony atoms, instead of similar size. In other words, similar-sized atoms would cause a mole of arsenic to be 63% larger than a mole of antimony, with a correspondingly lower density, allowing its volume to more closely mirror its estrus capacity behavior.
Issue of impurities [edit]
Sometimes small impurity concentrations can greatly affect the specific heat, for example in semiconducting ferromagnetic alloys.[32]
Specific heat of liquids [edit]
A general theory of the heat capacity of liquids has non yet been achieved, and is still an agile area of research. It was long idea that phonon theory is not able to explain the oestrus capacity of liquids, since liquids only sustain longitudinal, simply non transverse phonons, which in solids are responsible for 2/3 of the heat capacity. However, Brillouin scattering experiments with neutrons and with X-rays, confirming an intuition of Yakov Frenkel,[33] take shown that transverse phonons practice exist in liquids, albeit restricted to frequencies above a threshold chosen the Frenkel frequency. Since most energy is independent in these high-frequency modes, a elementary modification of the Debye model is sufficient to yield a expert approximation to experimental heat capacities of simple liquids.[34]
Because of high crystal binding energies, the effects of vibrational mode freezing are observed in solids more than often than liquids: for instance the estrus chapters of liquid water is twice that of ice at nearly the aforementioned temperature, and is again shut to the 3R per mole of atoms of the Dulong–Petit theoretical maximum.
Baggy materials tin can be considered a blazon of liquid at temperatures above the drinking glass transition temperature. Below the drinking glass transition temperature amorphous materials are in the solid (burnished) country class. The specific estrus has characteristic discontinuities at the glass transition temperature which are acquired by the absenteeism in the burnished land of percolating clusters fabricated of cleaved bonds (configurons) that are present only in the liquid phase.[35] Above the glass transition temperature percolating clusters formed past broken bonds enable a more floppy construction and hence a larger degree of liberty for atomic motion which results in a higher heat capacity of liquids. Beneath the glass transition temperature there are no extended clusters of cleaved bonds and the heat capacity is smaller because the solid-land (glassy) structure of baggy fabric is more than rigid. The discontinuities in the heat capacity are typically used to notice the glass transition temperature where a supercooled liquid transforms to a drinking glass.
Outcome of hydrogen bonds [edit]
Hydrogen-containing polar molecules like ethanol, ammonia, and water have powerful, intermolecular hydrogen bonds when in their liquid phase. These bonds provide another place where rut may be stored as potential energy of vibration, even at comparatively low temperatures. Hydrogen bonds account for the fact that liquid water stores nearly the theoretical limit of iiiR per mole of atoms, even at relatively low temperatures (i.e. about the freezing signal of h2o).
See also [edit]
- Breakthrough statistical mechanics
- Heat capacity ratio
- Statistical mechanics
- Thermodynamic equations
- Thermodynamic databases for pure substances
- Estrus equation
- Heat transfer coefficient
- Heat of mixing
- Latent heat
- Material properties (thermodynamics)
- Joback method (Estimation of heat capacities)
- Specific oestrus of melting (Enthalpy of fusion)
- Specific oestrus of vaporization (Enthalpy of vaporization)
- Volumetric oestrus chapters
- Thermal mass
- R-value (insulation)
- Storage heater
- Frenkel line
References [edit]
- ^ International Agency of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), ISBN92-822-2213-six, archived (PDF) from the original on 2021-06-04, retrieved 2021-12-16
- ^ W. Wagner, J. R. Cooper, A. Dittmann, J. Kijima, H.-J. Kretzschmar, A. Kruse, R. Mare, K. Oguchi, H. Sato, I. Stöcker, O. Šifner, Y. Takaishi, I. Tanishita, J. Trübenbach and Th. Willkommen (2000): "The IAPWS industrial formulation 1997 for the thermodynamic properties of water and steam", ASME J. Eng. Gas Turbines and Power, book 122, pages 150–182
- ^ International Wedlock of Pure and Applied Chemical science, Physical Chemistry Segmentation. "Quantities, Units and Symbols in Physical Chemical science" (PDF). Blackwell Sciences. p. 7.
The describing word specific before the proper noun of an extensive quantity is often used to hateful divided past mass.
- ^ Lange's Handbook of Chemistry, 10th ed. p. 1524
- ^ Shuen-Chen Hwang, Robert D. Lein, Daniel A. Morgan (2005). "Noble Gases". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. |doi=10.1002/0471238961.0701190508230114.a01.pub2
- ^ Hwang, Shuen-Cheng; Weltmer, William R. (2000). "Helium Group Gases". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01. ISBN0-471-23896-1.
- ^ Feynman, R., Lectures in Physics, vol. I, chapter twoscore, pp. vii–eight
- ^ Reif, F. (1965). Fundamentals of statistical and thermal physics . McGraw-Colina. pp. 253–254. ISBN9780070518001.
- ^ Charles Kittel; Herbert Kroemer (2000). Thermal physics. Freeman. p. 78. ISBN978-0-7167-1088-2.
- ^ Textbook: Young and Geller College Physics, 8e, Pearson Education, 2008
- ^ Steven T. Thornton and Andrew Male monarch (1993): Modern Physics for Scientists and Engineers, Saunders Higher Publishing, 1993
- ^ Quantum Physics and the Physics of large systems, Part 1A Physics, Academy of Cambridge, C.K. Smith, 2008.
- ^ a b "Nitrogen" NIST Chemical science WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ M.Due west. Chase Jr. (1998) NIST-JANAF Themochemical Tables, 4th Edition, In Journal of Physical and Chemical Reference Data, Monograph 9, pages i–1951.
- ^ "Hydrogen" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ "Carbon monoxide" NIST Chemical science WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ "Chlorine"" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ "Bromine" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ Smith, C. K. (2008). Breakthrough Physics and the Physics of big systems, Part 1A Physics. Academy of Cambridge.
- ^ "Nitrous oxide" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-xviii.
- ^ "Cyanogen" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-eighteen.
- ^ "Acetylene" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-eighteen.
- ^ "Water" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ "Methane" NIST Chemistry WebBook, SRD 69, online. Accessed on 2019-05-18.
- ^ "Ethane" NIST Chemistry WebBook, SRD 69
- ^ Kittel, Charles (2005). Introduction to Solid State Physics (8th ed.). Hoboken, New Bailiwick of jersey, USA: John Wiley & Sons. p. 141. ISBN978-0-471-41526-eight.
- ^ Blundell, Stephen (2001). Magnetism in Condensed Matter . Oxford Master Series in Condensed Matter Physics (1st ed.). Hoboken, New Jersey, USA: Oxford University Printing. p. 27. ISBN978-0-19-850591-iv.
- ^ Kittel, Charles (2005). Introduction to Solid State Physics (8th ed.). Hoboken, New Jersey, United states of america: John Wiley & Sons. p. 141. ISBN978-0-471-41526-viii.
- ^ "The Rut Capacity of a Solid" (PDF). Archived from the original (PDF) on 2014-02-11.
- ^ Petit A.-T., Dulong P.-50. (1819). "Recherches sur quelques points importants de la Théorie de la Chaleur". Annales de Chimie et de Physique. ten: 395–413.
- ^ Petit A.-T., Dulong P.-Fifty.: Recherches sur quelques points importants de la Théorie de la Chaleur. In: Annales de Chimie et de Physique 10, 395–413 (1819) (Translation)
- ^ Hogan, C. (1969). "Density of States of an Insulating Ferromagnetic Alloy". Physical Review. 188 (2): 870. Bibcode:1969PhRv..188..870H. doi:x.1103/PhysRev.188.870.
- ^ In his textbook Kinetic Theory of Liquids (engl. 1947)
- ^ Bolmatov, D.; Brazhkin, V. Five.; Trachenko, M. (2012). "The phonon theory of liquid thermodynamics". Scientific Reports. 2: 421. arXiv:1202.0459. Bibcode:2012NatSR...2E.421B. doi:x.1038/srep00421. PMC3359528. PMID 22639729.
- Hamish Johnston (13 June 2012). "Phonon theory sheds light on liquid thermodynamics". Physics World.
- ^ Ojovan, Michael I.; Lee, William Eastward. (2006). "Topologically matted systems at the glass transition" (PDF). Journal of Physics: Condensed Matter. eighteen (50): 11507–11520. Bibcode:2006JPCM...1811507O. doi:10.1088/0953-8984/xviii/50/007.
Molar Heat Capacity Of Water,
Source: https://en.wikipedia.org/wiki/Molar_heat_capacity
Posted by: gallardothercits.blogspot.com



0 Response to "Molar Heat Capacity Of Water"
Post a Comment